Work package 5
WP5 will employ in vitro, ex vivo and in vivo methodologies to elucidate the clinical potential of the advanced wound care materials in regards to safety, diagnostics, and wound healing. The safety and efficacy of treatment using the developed wound dressing materials has been evaluated using state-of-the art model systems, and the effect of the novel dressing material on three aspects has been evaluated; 1) proliferation of human keratinocytes and fibroblasts, 2) migration of human keratinocytes and fibroblasts, and 3) healing process of human tissue ex vivo wounds. Further evaluation of toxicology and safety has been performed using a porcine wound healing model. We have extensive experience of the model systems in the context of wound healing, dressing materials, infection, and transplantation, and a thorough understanding of potential and limitations. The work in work package 5 will prepare for the clinical validation of the novel wound dressings developed in the HEALiX project.
Cytocompatibility. In order to assess the safety of dressing material candidates (WP2), pH sensing materials (WP3), and antimicrobial peptides (WP4), several of the mentioned model systems have been used. Firstly, in vitro cell culture using primary cultures of human fibroblasts and keratinocytes have been used to investigate effects on proliferation and migration of skin cells. This has been achieved using both traditional cell culture as well as the Livecyte 2 kinetic cytometer to provide high resolution in vitro data. In general, all tested nanocellulose materials have demonstrated good cytocompatibility. Some optimizations of pH sensors and peptides have been necessary to achieve sufficient safety levels.
Ex vivo wound models. An infected ex vivo wound model based on healthy human skin has been developed and validated. The model has been used for both toxicity and efficacy studies. Sustainable infection well above the threshold of clinically relevant levels (105 colony forming units (CFU)/g of tissue) have been shown for the Staphylococcus aureus, Pseudomonas aeruginosa and Acinetobacter baumannii Additionally, dose-response curves for treating these infections with antibiotics have been established for future use of the model.
In vivo models. Topical application of PLNC8αβ has been evaluated using the porcine model. The results revealed different antimicrobial effects of PLNC8αβ enantiomeres. Following treatment, wounds were shown to heal during the time period of the study, strongly implying that the peptides are non-toxic. Ethical permission was obtained from the Regional Animal Ethics Committee.
Extensive model application is described in for example:
Olof Eskilson, Elisa Zattarin, Linn Berglund, et al. Nanocellulose composite wound dressings for real-time pH wound monitoring. Materials Today Bio, Volume 19, 2023,100574.
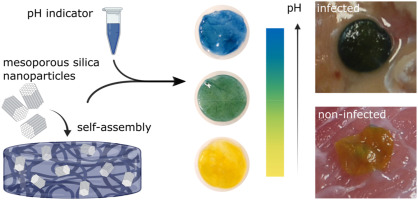
All HEALiX publications are found under “Publications” in the main menu.
Read more on the other work packages of HEALiX:
WP2 – Materials developpment and optimization
WP3 – Embedded wearable sensors
WP4 – Antimicrobial materials
WP6 – Clinical validation
